Introduction: Primary central nervous system lymphoma (PCNSL) is a rare, aggressive, non-hodgkin lymphoma, characterized by exclusive confinement to the central nervous system. Outcomes are poor, with a 5 year overall survival of 30% with standard induction of high-dose-methotrexate (HD-MTX) based regimens followed by consolidation. Barring epidemiologic trends due to immunodeficiency disorders, PCNSL is a disease of the immunocompetent older patient (age >60 years). Incidence in those aged >70 years is 10 times higher than the general population. The overall survival hasn't significantly changed in 4 decades. We present retrospective data in a rural, elderly population in Georgia.
Methods: We reviewed all patients diagnosed with PCNSL at our institution from January 1, 2013 to January 1, 2023. A retrospective chart review was conducted to assess for demographic factors, comorbidities, organ dysfunction, induction regimens, and consolidation treatments influencing patient care.
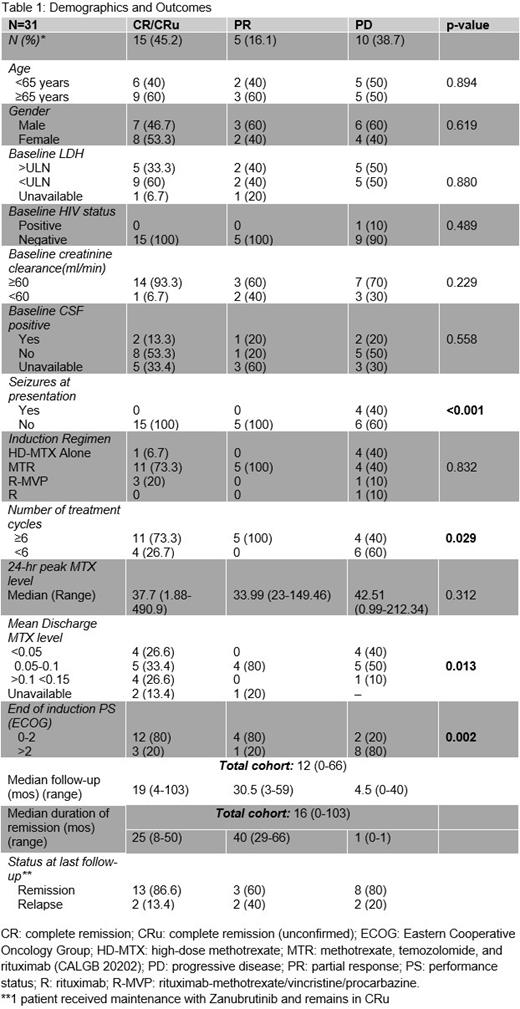
Results: A total of 38 PCNSL patients were identified. 6 died and one was lost to follow-up prior to start of therapy and are not further analyzed here. All patients had DLBCL histology. The median age was 62.3 (21-82 years) with 54.8% male. Patients identified as non-Hispanic white (77.4%), African-American (9.68%), Asian (6.46%), Hispanic or Latino (3.23%), and multiracial (3.23%). The youngest patient aged 21 years had HIV/AIDS. 2 of 3 patients diagnosed in their 30s were on immunosuppression, for renal transplant and multiple sclerosis. No patients had hepatitis B or C. Medical comorbidities were common with 80.7% of patients having at least one which included HTN, TIIDM, history of CVA or CAD, and atrial fibrillation. 19.4% of patients had impaired renal function (CrCl <60 ml/min).
Several patterns emerged when stratified by best treatment response at end of induction (EOI). Median follow-up was 12 mos (range: 0-66). Age, HIV status, gender, CrCl, positive CSF at baseline, or elevated LDH were not statistically significant predictors of response. This is probably due to the small sample size and missing data. Seizures at disease outset were associated with a statistically significant worse response to induction and all patients without seizures responded. This is likely a proxy for deep brain involvement and data are similar to CALGB 50202 where deep brain lesions independently correlated with a shorter progression-free-survival. Completion of ≥ 6 cycles of induction and a lower MTX level at discharge were predictors for response likely reflective of better organ function. Majority of patients with progressive disease received fewer than 6 cycles. A poorer EOI ECOG PS portended a worse response and all responders maintained an EOI PS of 0-2. Peak MTX level did not correlate with response. Sample size was too small to discriminate between discharge MTX thresholds of <0.05 vs 0.1 vs >0.15 however that will be an area of further investigation. Choice of induction regimen did not predict response statistically, however, all responders received HD-MTX-based combination chemotherapy (77.42%). 19.4% received HD-MTX alone and 3.2% received rituximab monotherapy. Only 8 (25.8%) of patients received consolidation. 7 received non-myeloablative therapy with 2 receiving additional WBRT and 1 patient received WBRT alone.
Conclusion: PCNSL remains an area of high unmet need. Recently, IELSG43 and MARTA studies have shown that HD-MTX-based combination chemoimmunotherapy followed by autologous stem cell transplant leads to improved outcomes regardless of age; however, non-relapse mortality remains a concern. Our data comprising chiefly of an elderly cohort reiterate efficacy of combination chemo-immunotherapy and impact of number of induction cycles on response, despite age. Most common reasons for treatment discontinuation included performance status deterioration and death from disease or toxicity. A multidisciplinary approach with physical therapy, nutritionist, neurology, and geriatrics would be optimal, particularly for older and infirm patients. In addition, alternatives to consolidation such as targeted agent (e.g. Bruton tyrosine kinase inhibitor) maintenance should be considered to improve outcomes in the elderly. One patient at our center is receiving post-induction zanubrutinib with a complete remission to date (~16 mos). This will be a focus for a phase II clinical trial at our institution.
Disclosures
Keruakous:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees. Cortes:Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Gilead: Consultancy; Takeda: Consultancy, Honoraria. Kota:Pfizer: Honoraria; Kite: Honoraria; Incyte: Research Funding; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal